
Kirinyaga County, showing cylindrical hind body (black arrow)
| Livestock Research for Rural Development 32 (10) 2020 | LRRD Search | LRRD Misssion | Guide for preparation of papers | LRRD Newsletter | Citation of this paper |
Parasitic infestation in fish can lead to severe retarded growth and may be accompanied by mortalities. Importantly, the quality of water in the holding facility may influence the parasitic biota. Therefore, a cross-sectional study was carried out from December 2017 to April 2018 in Kirinyaga County, Kenya, to determine the prevalence, intensity and the relationship between physico-chemical parameters of water and farmed fish parasites. A total of 294 live fish (Oreochromis niloticus, Clarias gariepinus, Carassius auratus and Cyprinus carpio carpio) were purchased from 22 randomly selected fish farms within the county. Physico-chemical parameters of water in 31 ponds were assessed in situ and ex situ, following standard procedures. Sampled fish were dissected immediately and subjected to parasitological examination by visual observation and light microscopy. The overall prevalence of parasitic infestation of the fish examined was 26.5% (78/294); with the highest infestation recorded in Nile tilapia (73.1%; 57/78). Eight parasite genera were recovered, with Diplostomum and Acanthocephalus species dominating each at 6.5% (19/294). The highest mean intensity was observed in Neascus spp. at 190 with an abundance range of 4-596 parasites. Mean physicochemical parameters of water were: pH (7.4), dissolved oxygen (5.9mgl-1), water temperature (25.3°C), phosphates (1.1mgl-1), nitrites (0.3mgl-1), nitrates (29.3mgl -1) and ammonia free nitrogen (0.8mgl-1). The water temperature and dissolved oxygen were below the optimal limit, while pH, ammonia, nitrites and phosphates levels in some ponds were above the desired limit for fish farming. Parasitic prevalence was positively correlated with ammonia free nitrogen, nitrates and phosphates, while there was a significant correlation between dissolved oxygen and abundance of the digenean trematode, Clinostomum cutaneum. These findings show that farmed fish harbour several parasite genera which may be influenced by physico-chemical characteristics of pond water. Fish farmers are advised to regularly monitor pond water quality in order to minimize fish stress and parasitic infestations; thus improving aquaculture productivity. Further long-term studies are recommended to determine the effect of water quality on parasitic fauna of farmed fish.
Key words: aquaculture productivity, Clinostomum cutaneum, Diplostomum, dissolved oxygen, Neascus
Warm freshwater aquaculture in Kenya encompasses food fish (Oreochromis niloticus and Clarias gariepinus) and ornamental fish (Carassius auratus and Cyprinus carpio carpio) farming. In the recent times; there has been remarkable growth in aquaculture with marked intensification. Even though, Kenya like most tropic countries has long periods of warm weather that is ideal for proliferation of ecto- and endo-parasites; a major risk of diseases comes from intensification.
Fish parasitic diseases are a result of imbalance in interaction between fish and parasite factors and aquatic environment (culture) conditions. However, occurrence and magnitude of these diseases is closely related to sanitary conditions in water (Hossain et al 2007). Any characteristic of water quality is therefore the most important key to a successful fish production. Any characteristic of water that affects the survival, reproduction, growth, or management of fish is a water quality variable (Noga 2010). It is postulated that water pollution influences the pathogenicity potential of ecto- and endo-parasites (Khan and Thulin 1991); while in fish the same poor water quality increases disease susceptibility (Biswas and Pramanik 2016), by lowering the defense and immunogenic status of the fish (Noor El-Deen et al 2015).
Environmental factors such as water temperature, dissolved oxygen, salinity, hydrogen ion concentration and eutrophication have a positive influence on the occurrence of parasitic populations and communities (Ali et al 2004; Lagrue et al 2011; Zargar et al 2012; Ojwala et al 2018). Stress is related to lower water quality (lower values of dissolved oxygen, inadequate values of temperature, pH, and high levels of organic matter in the water), which provide a good environment for proliferation of protozoan parasites and occurrence of pathogenic agents, mainly the body surface parasites like ecto-protozoans of fish (Paulista et al 2009).
Parasites are recognized as an important limiting factor in the development of intensified fish culture; where they result in massive mortalities and reduced growth rates (Iwanowicz 2011). Legion studies on parasitic infestations in culture fish in Kenya have been carried out antecedently (Migiro et al 2012; Khamis et al 2017; Mavuti et al 2017; Maina et al 2017; Murugami et al 2018; Mukwabi et al 2019; Wanja et al 2020); however most of these studies have focused on the systematics with little attention in determining the association of water quality parameters in influencing the presence of fish parasites. Under pond culture conditions, a number of natural abiotic factors, particularly from water quality may influence the presence of parasites. Thus, this study was conducted with the intention to determine occurrence of parasites and to evaluate the effect of physico-chemical characteristics of water on parasitic infestation in farmed fish in Kirinyaga County, central Kenya.
Prior to commencement of this study, ethical clearance was approved by the Biosafety, Animal Use, Care, and Ethics Committee of the Faculty of Veterinary Medicine, University of Nairobi. Verbal consent was also sought from fish farmers enumerated in this study.
A cross-sectional study that involved fish sampling for parasitological examination and pond water quality assessment was carried out from December 2017 to April 2018 in Kirinyaga County. Kirinyaga County is situated in central Kenya between 0°39'S latitude and 37°12'E longitude, at an elevation of 1230m above sea level. The county has five sub-counties namely: Mwea West, Mwea East, Kirinyaga West, Kirinyaga Central and Kirinyaga East. The county was purposively selected owing to high number of active pisciculturists (Anonymous 2018). A total of 22 fish farms were engaged and most farmers cultured tilapia in liner fish ponds. Parasitological examination was done by visual observations and by light microscopy, while water quality features was assessed in situ and ex situ at the time of sampling, following American Public Health Association (2008) guidelines.
Pond water quality was assessed in 31 ponds (the same ponds in which fish sampling was done) in the morning (between 0800 and 1000 hours) on the day of fish sampling. Physico-chemical parameters of water including pH, temperature and dissolved oxygen were measured in situ using a portable waterproof HANNA Multiprobe meters (Hanna Instruments Inc., USA) at three different points of the pond. Water samples were collected for spectrophotometry chemical analysis for; phosphates, nitrites, nitrates and ammonia free nitrogen at Government Chemist, Kenyatta National Hospital, Kenya.
A total of 294 fish samples were harvested using a seine net from 22 smallholder fish farms distributed across all the five sub-counties of Kirinyaga County. Of these, 162 were Nile tilapia (Oreochromis niloticus), 80 were African catfish (Clarias gariepinus), and the rest were ornamental fish [goldfish (Carassius auratus) and koi carp (Cyprinus carpio carpio)]. Live sampled fish together with source pond water were immediately transferred to the County Veterinary Laboratory, Kerugoya town for parasitological assay.
An external examination of individual live fish was performed visually to check for any ectoparasites and gross lesions according to methods described by Woo (2006) and Noga (2010). The examined fish were euthanized and post-mortem examination was done using standard necropsy procedures (Noga 2010; Roberts 2012). Briefly, a midline incision was made with a sterile scalpel blade starting at the anterior end of the anal opening to the operculum. A lateral cut from the anal opening on the abdominal wall of the fish up to the upper corner of the operculum was made to expose the abdominal organs. The body wall was then deflected and the organs observed grossly in situ using methods described by Amlacher (1970); any pathological lesions present were noted and recorded. A third cut connecting the two previous incisions at the operculum was made to remove the skin and muscular flap.
Wet mounts of skin scrapings and/or slime and gill filaments were obtained on slides on a drop of physiological saline and examined under light microscope for ectoparasites at X10 and X40 (Lucky 1977). The eyes contents were eviscerated on a clean slide and checked for ocular trematodes. The gastrointestinal tract was collected and preserved in 70% ethanol for parasitological examination. The contents were later emptied in a petri dish with normal saline (0.85% Sodium chloride) and examined for parasites using a dissecting microscope. Microscopic parasites were collected by a special needle; then washed for several times in warm saline solution and left in the refrigerator until the specimens had died and completely relaxed. The identification and characterization of parasites was performed using morphological features as described by others (Chubb et al 1987; Paperna 1996; Woo 2006; Noga 2010; Roberts 2012).
Collected data was validated, entered and stored in Microsoft Excel which was also used to calculate means and proportions. Parasitic infestations were evaluated using epidemiological parameters i.e., prevalence, mean abundance and mean intensity of infestation as described by Margolis et al (1982) and Bush et al (1997). Pearson’s correlation was used to establish the correlation co-efficient between different physico-chemical parameters and parasitic infestations.
Out of 294 fish sampled and examined, 26.5% (78/294) were infested by one or more parasite species. Eight fish parasite species were isolated, identified and recorded as; Diplostomum spp., Neascus spp., Clinostomum cutaneum, Gyrodactylus spp., Dactylogyrus spp., Acanthocephalus spp., Piscicola spp. and Pseudophylledian cestodes. The most prevalent parasite genera were Diplostomum and Acanthocephalus each at 6.5%, with a mean intensity of 32.7 and 4.4, respectively (Table 1). The highest mean intensity was observed in Neascus spp. at 190.3 with a parasite density of 4-596 (Table 1). Majority of the parasites were recovered from the skin, while the rest were infesting the gills, intestines/gut, eyes, fins and muscle. Of the 78 infested fish, 73.1% (57/78) were Nile tilapia, 15.4 % (12/79) ornamental fish and the rest were African catfish.
|
Table 1. Prevalence and mean intensity of parasitic infestation in different organs of sampled fish species from Kirinyaga County |
||||
|
Parasites |
Fish host |
Organ(s) infested |
Mean intensity |
Prevalence |
|
Digenean trematodes |
||||
|
Diplostomum spp. |
Tilapia, catfish |
Eyes |
32.7 (2-165) |
6.5 |
|
Clinostomum cutaneum |
Tilapia |
Skin |
4.6 (1-19) |
3.1 |
|
Neascus spp. |
Tilapia |
Skin, muscles, eyes, fins, gills |
190.3 (4-596) |
4.1 |
|
Monogenean worms |
||||
|
Gyrodactylus spp. |
Tilapia |
Skin |
2 (2) |
0.1 |
|
Dactylogyrus spp. |
Tilapia, catfish, goldfish, koi carp |
Gills |
1.7 (1-2) |
6.1 |
|
Cestodes |
||||
|
Pseudophylledian cestodes |
Tilapia, catfish |
Intestinal wall/gut |
2.8 (1-4) |
1.7 |
|
Spiny headed worms |
||||
|
Acanthocephalus spp. |
Tilapia, catfish, goldfish |
Intestinal wall/gut |
4.4 (1-8) |
6.5 |
|
Leeches |
||||
|
Piscicola spp. |
Tilapia |
Skin |
2 (2) |
0.1 |
Diplostomum spp. were identified by their cylindrical hind body containing the immature gonads (Figure 1) and were found actively swimming alive in the vitreous humor of examined Nile tilapia and African catfish.
 |
| Figure 1. Diplostomum spp. recovered from Nile tilapia and African
catfish in Kirinyaga County, showing cylindrical hind body (black arrow) |
Clinostomum cutaneum appeared as yellowish grubs/cysts on the skin of Nile tilapia. They were dorso-ventrally flattened, oval, with a sucker around the anterior mouth and an acetabulum. They had a digestive system consisting of a pharynx connected to the mouth opening and a short esophagus and two blind intestinal caeca (Figure 2).
 |
| Figure 2. Yellow grubs on the skin (red arrows) and metacercariae of Clinostomum cutaneum recovered from muscles of Nile tilapia (white stars shows intestinal ceca of metacercariae) |
Metacercaria of the genus Neascus appeared as black grub/spots averaging 1-2mm in diameter on the fins, mouth, skin, gills and eyes in Nile tilapia (Figure 3); with no observable/significant post-mortem findings.
 |
| Figure 3. Multifocal black spots on the tail fin (red arrows) of Nile tilapia due to Neascus spp. metacercariae infestation |
Adult worms of the genus Dactylogyrus were isolated from gills of Nile tilapia. They were recognized by the prohaptor bearing the four-lobed head with four eye spots and reproductive organs Gyrodactylus spp. were recovered from the skin of tilapia and identified by their anchor hooks at the posterior end.
Acanthocephalus spp. were recovered from intestines of a tilapia and identified by their characteristic protruding proboscis (Figure 4).
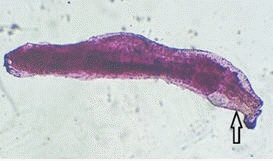 |
| Figure 4. Acanthocephalus spp. showing spiny proboscis (arrow)
recovered from intestines of Oreochromis niloticus in Kirinyaga County |
Piscicola spp. are macro-ectoparasites which were found attached to the skin of Nile tilapia. The leeches were identified by anterior/oral and caudal suckers with a sub-cylindrical elongate body. Piscicola spp. infested fish had excess mucus grossly with pale friable liver at necropsy (Figure 5). There were no significant findings seen in other visceral organs.
 |
| Figure 5. Detached Piscicola leeches (black arrows) recovered from Nile tilapia whose liver was pale and friable (inset) |
Cestodes were identified by their segmented body (proglottids) and an anterior attachment organ (scolex) (Figure 6).
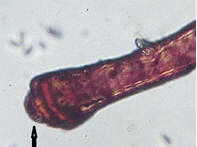 |
| Figure 6. A cestode showing sucker (black arrow) recovered from intestines of Oreochromis niloticus in Kirinyaga County |
The mean and range values of some physico-chemical parameters of 31 pond water samples, against recommended limits for fish culture are tabulated in Table 2. Most ponds evaluated had water temperature below the recommended limit for fish rearing; while a few had pH levels above the recommended limit. Levels of nitrates were within the optimum range for pisciculture (Table 2).
|
Table 2. The mean of physicochemical characteristics of pond water in Kirinyaga County |
||||
|
Parameter |
Mean (range) |
Recommended |
Percentage (%) above or |
|
|
Temperature (0C) |
25.3 (20.5 – 31.7a) |
26 – 32 |
58.1% (n = 18) |
|
|
Dissolved oxygen (mgl-1) |
5.9 (0.13 – 8.4a) |
3 – 5 |
22.5% (n = 7) |
|
|
pH |
7.4 (6.5 – 9.2b) |
6.5 – 8.5 |
6.5% (n = 2) |
|
|
Ammonia free nitrogen (mgl-1) |
0.8 (0 – 9.9b) |
0 – 0.2 |
32.3% (n = 10) |
|
|
Nitrites (mgl-1) |
0.3 (0 – 3.1b) |
0 – 0.2 |
16.1% (n = 5) |
|
|
Nitrates (mgl-1) |
29.3 (0 – 113) |
0 – 200 |
0% (n = 0) |
|
|
Phosphates (mgl-1) |
1.12 (0 – 23.6b) |
0.03– 2 |
35.5% (n = 11) |
|
|
aOnly lower value was below the optimum range;
bOnly upper value was above the optimum range;
|
||||
Table 3 shows the water quality parameters against prevalence of parasites in that particular pond and their respective correlation coefficient and interpretation. Parasitic prevalence was positively correlated with ammonia free nitrogen, nitrates and phosphates in the study area (Table 3).
|
Table 3. Correlation coefficients of total parasite prevalence rates to water parameters and their interpretation |
||
|
Water parameter |
Correlation |
Nature of |
|
Temperature |
-0.139 |
Negligible |
|
Dissolved oxygen |
-0.406 |
Low negative |
|
pH |
-0.343 |
Low negative |
|
Ammonia free nitrogen |
0.582 |
Moderate positive |
|
Nitrates |
0.286718 |
Low positive |
|
Nitrites |
-0.0633 |
Negligible |
|
Phosphates |
0.412486 |
Low positive |
|
*Nature of association adopted from Mukaka (2012) |
||
The correlation matrix between some independent variables (temperature, dissolved oxygen, pH and ammonia free nitrogen), and the dependent variable (parasitic abundance) is given in Table 4. There was a significant positive correlation (r = 0.5; p<0.05) between dissolved oxygen and Clinostomum cutaneum abundance (Table 4).
|
Table 4. Correlation coefficients between abundance of fish parasites and physico-chemical parameters of pond water |
|||||
|
Fish parasites |
Physico-chemical parameters of water |
||||
|
Water |
Dissolved |
pH |
Ammonia |
||
|
Diplostomum spp. |
-0.2 |
-0.4 |
-0.3 |
-0.1 |
|
|
Clinostomum cutaneum |
0.4 |
0.5* |
0.5 |
-0.1 |
|
|
Neascus spp. |
0.2 |
-0.05 |
0.22 |
-0.06 |
|
|
Gyrodactylus spp. |
0.2 |
0.2 |
0.3 |
-0.1 |
|
|
Dactylogyrus spp. |
0.02 |
0.03 |
0.07 |
-0.2 |
|
|
Acanthocephalus spp. |
0.05 |
0.04 |
0.17 |
-0.1 |
|
|
Piscicola leeches |
0.2 |
0.2 |
0.3 |
-0.1 |
|
|
Pseudophylledian cestodes |
0.2 |
0.3 |
0.2 |
-0.1 |
|
|
*Correlation significant at 0.05 (two-tailed) |
|||||
In Kenya, more attention is being focused nowadays to the intensification and commercialization of aquaculture to mitigate the dearth of aquatic protein. There is little effort towards fish health especially diseases. This is despite the fact that, fish diseases including parasitic ones can inevitably exerts deleterious effects ranging from extremely damaging to insignificant change on the host.
Fish parasites were isolated in 26.5% of the fish collected from smallholder fish farms in Kirinyaga County. This finding was similar to a study by Maina et al (2017) that investigated occurrence of parasites in farmed Nile tilapia and African catfish in Kiambu County, Kenya. Parasitic infestations were found in all the four fish species examined with a higher prevalence recorded in Oreochromis niloticus compared to Clarias gariepinus, Carassius auratus and Cyprinus carpio carpio. A similar finding was reported by Mavuti et al (2017); in a study investigating ecto- and endo-parasitosis in farmed tilapia and catfish in Nyeri County. Higher infestation among tilapia may be due to high stocking density which is a common practice in tilapia culture and therefore may facilitate rapid propagation of parasites. In the present study; monogeneans, digenean trematodes, acanthocephalans, cestodes and leeches were recovered from examined farmed fish from Kirinyaga County.
Three digenean trematodes were recorded in this study; Diplostomum spp., metacercaria of Neascus spp. and Clinostomum cutaneum. Previous studies have reported occurrence of Diplostomum spp. and metacercariae of Clinostomum cutaneum (Gustinelli et al 2010; Mavuti et al 2017; Murugami et al 2018). Diplostomum spp. was found swimming in the vitreous humor of both catfish and tilapia without causing major pathological defects. However, higher number of Diplostomum worms in the eye of the host may result to blindness and are more prone to predation (Seppala et al 2005).
Metacercariae of Neascus spp. were observed in the skin, muscles, eyes, fins, gills of tilapia at a prevalence of 4.1% and a mean intensity of 190 cysts per infested fish. These findings are in disagreement with those of Thon et al (2019) who reported a prevalence of 1% and mean intensity of 1 in the skin of tilapia in Winam Gulf of Lake Victoria, Kenya. Skin metacercariae of C. cutaneum were isolated from tilapia only at a prevalence of 3.1%. This finding is partially in line with Gichohi (2010) and Mavuti et al. (2017); who reported this parasite in farmed tilapia, however at higher prevalence in both studies. Clinostomum cutaneum was isolated in both fish and piscivorous birds (grey heron) in recent studies undertaken in Sagana Aquaculture Center, Kirinyaga County (Gustinelli et al 2010; Murugami et al (2018). Metacercariae of Neascus spp. and C. cutaneum causes black spot and yellow grub diseases, respectively. The resulting diseases make the fish unsightly thereby causing economic losses due to rejection (Lane and Morris 2010). Human cases of C. complanatum infestation have been reported in Japan (Hara et al., 2014) and Korea (Chung et al 1995; Kim et al 2019) and hence a public health concern.
The monogeneans recovered in this study were; Dactylogyrus and Gyrodactylus species and these findings are similar to those of Mavuti et al (2017). However, Dactylogyrus spp., a gill parasite was dominating; being isolated in all the four fish species examined. Gyrodactylus spp. which is generally found on the skin and fins of fish was recovered from tilapia only. This is contrary to a study by Mavuti et al (2017) and Murugami et al (2018) in which Gyrodactylus species was found in catfish only. Gyrodactylus and Dactylogyrus species are the two most common genera of monogeneans that infest freshwater fish with cultured tilapia being more susceptible compared to wild ones (Lim et al 2016). Monogeneans are important pathogens in aquaculture systems where fish are in high stocking densities and in confined environments (Hecht et al 1998). They propagate rapidly and are readily transmitted among fish and, if left uncontrolled high morbidity and mortality can occur in farmed fish leading to serious economic loss to farmers (Shinn et al 2015).
Acanthocephalus species were isolated from intestinal wall of tilapia, catfish and goldfish with overall prevalence of 6.5%; contrary to the findings of Mavuti et al (2017) and Kamundia (2012) who observed the worms in tilapia only and at lower infestation rate. The findings also contradicts studies of Aloo (2002) and Akoll et al (2012) who all reported it encysting in the intestinal wall and in the liver of tilapia. Ingestion of isopods, amphipods, and/or copepods by the fish has been attributed with acanthocephalan infestations (Paperna 1996; Ngasepam et al 2015). In heavily infected fish acanthocephalans may perforate the gut wall with their proboscis and cause considerable damage with severe local inflammatory reaction (Jithendran and Kannappan 2010).
Cestodes of the family Pseudophyllidae were recovered from the intestines of tilapia and catfish with an overall prevalence of 1.7%. This is contrary to work by Murugami et al (2018); where Pseudophyllidean and Proteocephallid cestodes were isolated from catfish.
Piscicola leeches were observed attaching on the skin of tilapia at a prevalence of 0.1%. This is contrary to findings by Mavuti et al (2017); where Piscicola leeches were recorded in the gills of both catfish and tilapia at an overall prevalence of 2.7%. Leeches are rare in cultured fish but are occasionally seen in wild fish (Noga, 2010). These ecto-parasites are blood suckers and are vectors of fish haematozoans (Kamundia 2012). This explains the observed gross and post-mortem finding of a pale friable liver; an indication of haemolytic anemia.Water quality is an important factor when it comes to fish health management. Of the 31 fish pond water samples assessed in this study; 11 (36%), 10 (33%), 5 (16%) and 2 (6.5%) exceeded the recommended limits of < 0.03-2 mgl-1, <0.2 mgl-1, < 0.02 mgl -1 and <8.5 of phosphates, ammonia free nitrogen, nitrites and pH, respectively. High levels of phosphates and nitrates could be attributed to chemical fertilization of crops within the vicinity of the pond. The soluble chemicals may still end up into the pond through leaching from the soil into water. High ammonia levels may be due to overfeeding of fish especially with highly proteinous feeds or high pond stocking densities which may lead to build up of nitrogenous wastes (mainly ammonia) from fish. The slightly higher pond water pH (slightly alkaline) may be attributed to soil alkalinity and/or pond liming. Water temperature and dissolved oxygen levels in 58.1% and 22.5% of the pond water samples, respectively, were below the optimum limit for fish rearing. Kirinyaga County has a tropical climate which is influenced by its position on the equator and the Mount Kenya. Temperature ranges from 8.1 to 30.3°C (Anonymous 2018). Levels of dissolved oxygen are positively influenced by temperature (Bhatnagar and Devi 2013).
In the present study, occurrence of parasites was positively correlated with ammonia free nitrogen, nitrates and phosphates. The findings are partially in line with that of Ojwala et al (2018) who reported a positive correlation of various parasitic assemblage and some physicochemical characteristics of pond water including; nitrates and soluble reactive phosphates. However, the present findings are contrary with those of Puinyabati et al (2013), who reported a positive correlation of prevalence of trematode parasites in Channa punctata (spotted snakehead) with dissolved oxygen, pH, conductivity and water temperature. Abiotic factors particularly ones inclined to pond water characteristics have been recognized as an important bottleneck factors in the biological activity of the culture facility (Ngasepam et al 2015). For instance, water quality attributes have been associated with occurrence of digenean trematodes and other parasites (Chubb 1979). This study observed that dissolved oxygen and intensity of C. cutaneum were correlated positively (p<0.05) and this finding has not been reported in Kenya before. However, the findings are contrary to those of Zargar et al (2012).
The authors have no conflict of interest, financial or otherwise, regarding this publication.
We wish to acknowledge the Norwegian Agency for Development Cooperation (NORAD) for financing this work under the capacity building for Training and Research in Aquatic and Environmental Health in Eastern and Southern Africa (TRAHESA) for financing this study. We thank the fisheries extension officers for assistance in locating farms; and fish farmers for their cooperation.
Akoll P, Konency R, Mwanja, W W and Schiemer F 2012 Infections patterns of Nile tilapia (O. niloticus L.1758) by two helminths species with contrasting life styles. Parasitology Research 110: 1461-1472.
Ali F M, Abdus–Salam B A, Ahmad K S, Qamar M and Umer K 2004 Seasonal variations of physico – chemical characteristics of River Soan water at Dhoak Pathan Bridge (Chakwal), Pakistan. International Journal of Agriculture and Biology 6(1): 89–92.
Aloo P A 2002 A comparative study of helminths parasites from the fish Tilapia zillii and Oreochromis leucostictus in Lake Naivasha and Oloidien Bay, Kenya. Journal of Helminthology 76: 95-102.
American Public Health Association 2008 Standard Methods for Examination of Water and Waste Water, American Public Health Association, Washington, DC, USA, 21st edition.
Amlacher E 1970 Text book of fish diseases T. F. H. publication, New Jersy, U.S.A., pp. 117-135.
Anonymous 2018 County Government of Kirinyaga, County Integrated Development Plan 2018-2022.
Bhatnagar A and Devi P 2013 Water quality guidelines for the management of pond fish culture. International Journal of Environmental Sciences, 3(6): 1980-2009.
Biswas J K and Pramanik S 2016 Assessment of aquatic environmental quality using Gyrodactylus sp. as a living probe: parasitic biomonitoring of ecosystem health. Journal of Advances in Environmental Health Research 4(4): 219-226.
Boyd C E 1982 Water quality management for pond fish culture. Elsevier, Amsterdam, 318.
Bush A O, Lafferty K D, Lotz J M and Shostak A W 1997 Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology 83: 575–583.
Chubb J C 1979 Seasonal occurrence of helminth parasite in fishes. Part-II. Trematoda. Advances in Parasitology 17: 171-313.
Chubb J C, Pool D W and Veltkamp C J 1987 A key to the species of cestodes (tapeworms) parasitic in British and Irish freshwater fishes. Journal of Fish Biology 31(4): 517-543.
Chung D I, Moon C H, Kong H H, Choi D W and Lim D K 1995 The first human case of Clinostomum complanatum (Trematoda: Clinostomidae) infection in Korea. The Korean Journal of Parasitology 33(3): 219–223.
Gichohi C M 2010 Prevalence, Intensity and Pathological lesions associated with Helminth infections in farmed and wild fish in Upper Tana River basin, Kenya. MSc. Thesis, University of Nairobi, Kenya.
Gustinelli A, Caffara, M, Florio D, Otachi E O, Wathuta E M and Fioravanti M L 2010 First description of the adult stage of Clinostomum cutaneum Paperna, 1964 (Digenea: Clinostomidae) from grey herons Ardea cinerea L. and a redescription of the metacercaria from the Nile tilapia Oreochromis niloticus niloticus (L.) in Kenya, Systematic Parasitology, 76(1): 39–51.
Hara H, Miyauchi Y, Tahara S and Yamashita H 2014 Human laryngitis caused by Clinostomum complanatum, Nagoya Journal of Medical Science 76: 109-113.
Hecht T and Endemann F 1998 The impact of parasites, infections and diseases on the development of aquaculture in sub-Saharan Africa. Journal of Applied Ichthyology. 14(3-4): 213-221.
Hossain M D, Hossain M K and Rahman M H 2007 Water quality parameters and incidence of fish diseases in some water bodies in Natore, Bangladesh. Journal of Life and Earth Science 2(2): 27–30.
Iwanowicz D D 2011 Overview on the Effects of parasites on fish health. United States Geological Survey, Leetown Science Center, National Fish Health Research Laboratory, 11649 Leetown Road, Kearneysville, WV 25430.
Jithendran K P and Kannappan S 2010 A short note on heavy infection of acanthocephalan worm (Neoechinorhynchus agilis) in grey mullet, Mugil cephalus. 34(2): 99–101.
Kamundia P W 2012 Pathological changes associated with pollution and endoparasites in Nile perch and tilapia in Lake Victoria, Kenya. MSc. Thesis, University of Nairobi, Kenya.
Khamis H M, Ali A I, Lusweti D and Orina P S 2017 Prevalence and diversity of internal cestode parasites of the Nile tilapia (Oreochromis niloticus) and African catfish (Clarias gariepinus) in different altitudes and ponds in Kenya. International Journal of Innovative Research and Advanced Studies 4(5): 220-224.
Khan R A and Thulin J 1991 Influence of pollution on parasites of aquatic animals. Advances in Parasitology 30: 202–238.
Kim H, Cho S W, Oh H, Byeon H K 2019 A case of unexpected Clinostomum complanatum infection initially presenting as foreign body in pharynx. The Korean Journal of Parasitology, 7(2): 175-177.
Lagrue C, Kelly D W, Hicks A and Poulin R 2011 Factors influencing infection patterns of trophically transmitted parasites among a fish community: host Diet, host–parasite compatibility or both? Journal of Fish Biology 79: 466-85.
Lane R L and J. E. Morris J E 2010 Biology, prevention, and effects of common grubs (digenetic trematodes) in freshwater fish. NCRAC Technical Bulletins, Iowa State, 2010.
Lim S Y, Ooi A L and Wong W L 2016 Gill monogeneans of Nile tilapia (Oreochromis niloticus) and red hybrid tilapia (Oreochromis spp.) from the wild and fish farms in Perak, Malaysia: infection dynamics and spatial distribution, SpringerPlus. Springer International Publishing 5(1609): 1–10.
Lucky Z 1977 Methods for the diagnosis of fish diseases, Amerind Publishing Co., New Delhi, India.
Maina K W, Mbuthia P G, Waruiru R M, Nzalawahe J, Murugami J W, Njagi L W, Mdegela R H and Mavuti S K 2017 Risk factors associated with parasites of farmed fish in Kiambu County, Kenya. International Journal of Fisheries and Aquatic Studies 5(4): 217-223.
Margolis L, Esch G W, Holmes J C, Kuris A M and Schad G A 1982 The use of ecological terms in parasitology (report Bush et al. – Parasite Ecology and Terminology 583 of an adhoc committee of the American Society of Parasitologists). Journal of Parasitology 68: 131–133.
Mavuti S K, Waruiru R M, Mbuthia P G, Maina J G, Mbaria J M and Otieno R O 2017 Prevalence of ecto- and endo-parasitic infections of farmed tilapia and catfish in Nyeri County, Kenya. Livestock Research for Rural Development. Volume 29, Article #122. Retrieved July 4, 2017, from http://www.lrrd.org/lrrd29/6/stek29122.htm
Migiro K E, Matolla G K, Ouko O V, Victor N M 2012 Diplostomum parasites affecting Oreochromis niloticus in Chepkoilel fish farm and two dams in Eldoret-Kenya. International Journal of Scientific and Engineering Research 3(7): 3-9.
Mukaka M M 2012 Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Medical Journal 24(3): 69–71.
Mukwabi D M, Otieno S O, Okemo P O, Odour R O and Agwanda B. 2019 Parasites infesting Nile tilapia grown in aquaculture systems in Kenya. Livestock Research for Rural Development. Volume 31, Article #20. Retrieved June 8, 2020, from http://www.lrrd.org/lrrd31/2/dmmak31020.html
Murugami J W, Waruiru R M, Maina K W, Mbuthia P G, Thaiyah A G, Mavuti S K, Otieno R O, Ngowi H A and Mdegela R H 2018 Helminth parasites of farmed fish and water birds in Kirinyaga County, Kenya. International Journal of Fisheries and Aquatic Studies 6(3): 06-12.
Ngasepam R S, Shomorendra M and Kar D 2015 Effect of physico-chemical parameters of water on abundance of trematode parasites of Channa punctata in Pumlen Lake, Manipur, India. TTPP, 443.
Noga E J 2010 Fish disease Diagnosis and Treatment. Mosby-yearbook, Inc. watsworth publishing Co., USA. pp.366.
Noor El-Deen A I, Abd El Hady O K, Kenawy A M, Mon A M and Mona S Z 2015 Study of prevailing external parasitic diseases in cultured freshwater tilapia Oreochromis niloticus. Life Science Journal 12(8): 30-37.
Ojwala R A, Otachi E O and Kitaka N K 2018 Effect of water quality on the parasite assemblages infecting Nile tilapia in selected fish farms in Nakuru County, Kenya. Parasitology Research 117(11): 3459-3471.
Paperna I 1996 Parasite infection and disease of fishes in Africa. An Update CIFA Technical paper, No. 31, Rome, FAO.
Paulista N, Postal A C and Federal U 2009 Protozoan parasites of Xiphophorus spp. (Poeciliidae) and their relation with water characteristics. Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 5: 156–162.
Puinyabati H, Shomorendra M and Kar D 2013 Correlation of water’s physico-chemical characteristics and trematode parasites of Channa punctata (Bloch) in Awangsoi lake, Manipur, India. Journal of Applied and Natural Science 5(1): 190-193.
Robert J R 2012 Fish Pathology. Fourth Edition. Edited by J R Robert. Wiley-Blackbell, West Sussex UK.
Seppala O, Karvonen A and Valtonen E. T 2005 Manipulation of fish host by eye flukes in relation to cataract formation and parasite infectivity. Animal Behaviour 70(4): 889–894.
Shinn A, Pratoomayot J, Bron J, Brooker A and Brooker E 2015 Economic impacts of aquatic parasites on global finfish production. Global Aquaculture Advocate, pp. 58-61.
Thon C C, Otachi O E and Oldewage A A 2019 Endo-helminths infestation in Nile Perch, Lates niloticus, (L.,) and Nile Tilapia, Oreochromis niloticus (L.,) in Winam Gulf of Lake Victoria, Kenya. Egerton Journal of Science and Technology 16(1). 1-139.
Wanja D W, Mbuthia P G, Waruiru R M, Mwadime J M, Bebora L C, Nyaga P N and Ngowi H A 2020 Fish Husbandry Practices and Water Quality in Central Kenya: Potential Risk Factors for Fish Mortality and Infectious Diseases. Veterinary Medicine International. Volume 2020 |Article ID 6839354 | 10 pages | https://doi.org/10.1155/2020/6839354
Woo P T K 2006 Fish diseases and disorders volume 1 protozoan and metazoan infections. 2nd edition. Edited by W P T K. University of Guelph, Canada.
Zargar U R, Yousuf, A R, Chishti M Z, Ahmed F, Bashir H and Ahmed F 2012 Effects of water quality and trophic status on helminth infections in the cyprinid fish, Schizothorax niger Heckel, 1838 from three lakes in the Kashmir Himalayas. Journal of Helminthology 86(01): 70–76.